Potential of Biochar as Cost Effective Adsorbent in Removal of Heavy Metals Ions From Aqueous Phase: A Mini Review
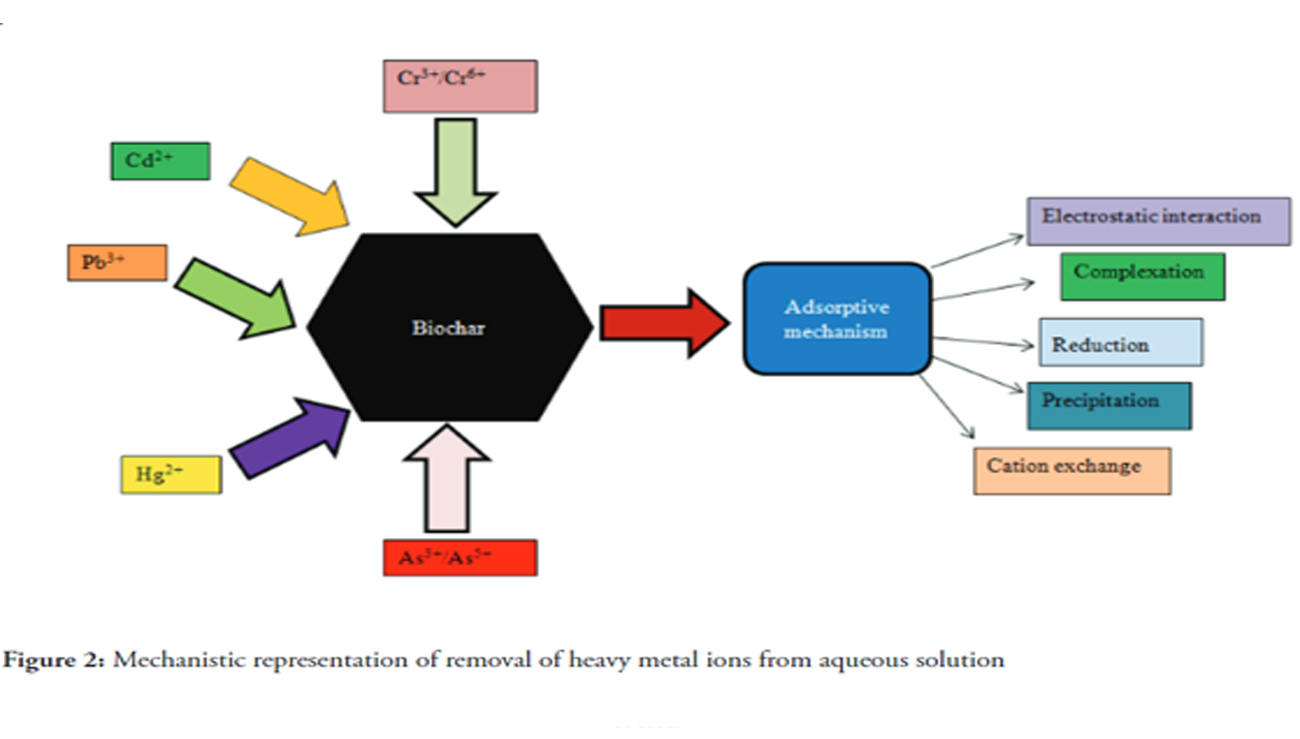
Due to industrialization and increasing population, wastewater treatment has become a big challenge. There are numerous techniques such as ion-exchange, adsorption, membrane filtration, coagulation, flocculation, floating and electrochemical approach developed for the remediation of contaminants from wastewater. But, now it is necessary to develop an approach which should has high efficiency, less expensive and environmental friendly, so that limitation of existing techniques can be overcome. Recent developments of biochar have attracted the researchers into this area. Different methods are discovered to synthesized biochar for the removal of pollutants from wastewater. In this review, biochar are elaborated and critically discussed which have reported for the removal of metallic pollutants present in waste water.
Author(s):
Keywords:
Adsorption, Biochar, Heavy Metals Ions, Contaminated Water, Purification
URL:
References:
Agrafioti, E., Kalderis, D. & Diamadopoulos, E. (2014). Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag., 133, 309–314. https://doi.org/10.1016/j.jenvman.2013.12.007
Ahmad, Z., Gao, B., Mosa, A., Yu, H., Yin, X., Bashir, A., Ghoveisi, H. & Wang, S. (2018). Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassiumrich biomass. J. Clean. Prod., 99, 19–23. https://doi.org/10.1016/j.jclepro.2018.01.133
Cao, X., Ma, L., Gao, B. & Harris, W. (2009). Dairymanure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol., 43, 3285–3291. https://doi.org/10.1021/es803092k
Demim, S., Drouiche, N., Aouabed, A., Benayad, T., Dendene-Badache, O. & Semsari, S. (2016). Cadmium and nickel: Assessment of the physiological effects and heavy metal removal using a response surface approach by L. gibba. J. Ecolog. Eng., 61, 426-435. https://doi.org/10.1016/j.ecoleng.2013.10.016
Dong, H., Deng, J., Xie, Y., Zhang, C., Jiang, Z., Cheng, Y., Hou, K. & Zeng, G. (2017). Stabilization of nanoscalezerovalent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater., 332, 79–86. https://doi.org/10.1016/j.jhazmat.2017.03.002
Gleick, P.H. (2003). Global freshwater resources: softpath solutions for the 21st century. Science, 302, 1524–1528. https://doi.org/10.1126/science.1089967
Hollister, C.C., Bisogni, J.J. & Lehmann, J. (2013). Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (L.) and oak wood (spp.). J. Environ. Qual., 42(1), 137–144. https://doi.org/10.2134/jeq2012.0033
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A.R., Pullammanappallil, P. & Cao, X. (2012). Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresource Technology, 110, 50–56. https://doi.org/10.1016/j.biortech.2012.01.072
Jin, H., Capareda, S., Chang, Z., Gao, J., Xu, Y. & Zhang, J. (2014). Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol., 169, 622–629. https://doi.org/10.1016/j.biortech.2014.06.103
Keiluweit M. & Kleber M. (2009). Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic pi-Systems. Environ. Sci. Technol., 43(10), 3421–3429. https://doi.org/10.1021/es8033044
Kim, W.K., Shim, T. Kim, Y.S., Hyun, S., Ryu, C., Park, Y.K. & Jung, J. (2013). Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour. Technol., 138, 266–270. https://doi.org/10.1016/j.biortech.2013.03.186
Kołodyńskaa R.D., Wnętrzak, R., Leahy, J.J., Hayes, M.H. B., Kwapiński, W. & Hubicki, Z. (2012). Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J., 197, 295–305. https://doi.org/10.1016/j.cej.2012.05.025
Kumar, S., Loganathan, V.A., Gupta, R.B. & Barnett, M.O. (2011). An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag., 92(10), 2504–2512.
https://doi.org/10.1016/j.jenvman.2011.05.013
Mahmoodi, N.M., Taghizadeh, M., Taghizadeh, A., Abdi, J.H., Bagher & Shekarchi, A.A. (2019). Biobased magnetic metal-organic framework nanocomposite: Ultrasound-assisted synthesis and pollutant (heavy metal and dye) removal from aqueous media. Appl. Surf. Sci., 480, 288–299. https://doi.org/10.1016/j.apsusc.2019.02.211
Mukherjee, A., Zimmerman, A.R., and Harris, W. (2011). Surface chemistry variations among a series of laboratory-produced biochars. Geoderma., 163(3-4), 247–255. https://doi.org/10.1016/j.geoderma.2011.04.021
Pap, S., Bezanovic, V., Radonic, J., Babic, A., Saric, S., Adamovic, D., Sekulic, M.T. (2018). Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq., 268, 315–325. https://doi.org/10.1016/j.molliq.2018.07.072
Saleh, M.E., El-Refaey, A.A. & Mahmoud, A.H. (2016). Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: a comparative study. Soil and Water Research, 11, 53–63. https://doi.org/10.17221/274/2014-SWR
Sun, K., Keiluweit, M., Kleber, M., Pan, Z. & Xing, B. (2011). Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Bioresour. Technol., 102(21), 9897–9903. https://doi.org/10.1016/j.biortech.2011.08.036
Usman, A., Sallam, A., Zhang, M., Vithanage, M., Ahmad, M., Al-Farraj, A., Ok, Y.S., Abduljabbar, A. & Al-Wabel, M. (2016). Sorption Process of Date Palm Biochar for Aqueous Cd (II) Removal: Efficiency and Mechanisms. Water Air Soil Pollut, 227, 449–460. https://doi.org/10.1007/s11270-016-3161-z
Wan, S., Wu, J., Zhou, S., Wang, R., Gao, B. & He, F. (2017). Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: Behavior and mechanism. Sci. Total Environ., 616-617, 1298–1306. https://doi.org/10.1016/j.scitotenv.2017.10.188
Wang, S., Gao, B., Li, Y., Mosa, A., Zimmerman, A. R., Ma, L. Q., Harris, W. G. & Migliaccio, K. W. (2015). Manganese oxide-modified biochars: preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol., 181, 13–17. https://doi.org/10.1016/j.biortech.2015.01.044
World Health Organization. Drinking-Water Fact-Sheet. http://www.who.int/mediacentre/factsheets/fs391/en/.
WWAP (United Nations World Water Assessment Programme). Water for a Sustainable World; The United Nations World Water Development Report: UNESCO:Paris 2015; pp 1−67.
Zhang, Y.P., Adi, V.S.K., Huang, H.L., Lin, H.P. & Huang, Z.H., 2019. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: adsorption isotherm and process simulation. J. Ind. Eng. Chem., 76, 240–244. https://doi.org/10.1016/j.jiec.2019.03.046