Role of Protein Kinase C in Diabetic Complications
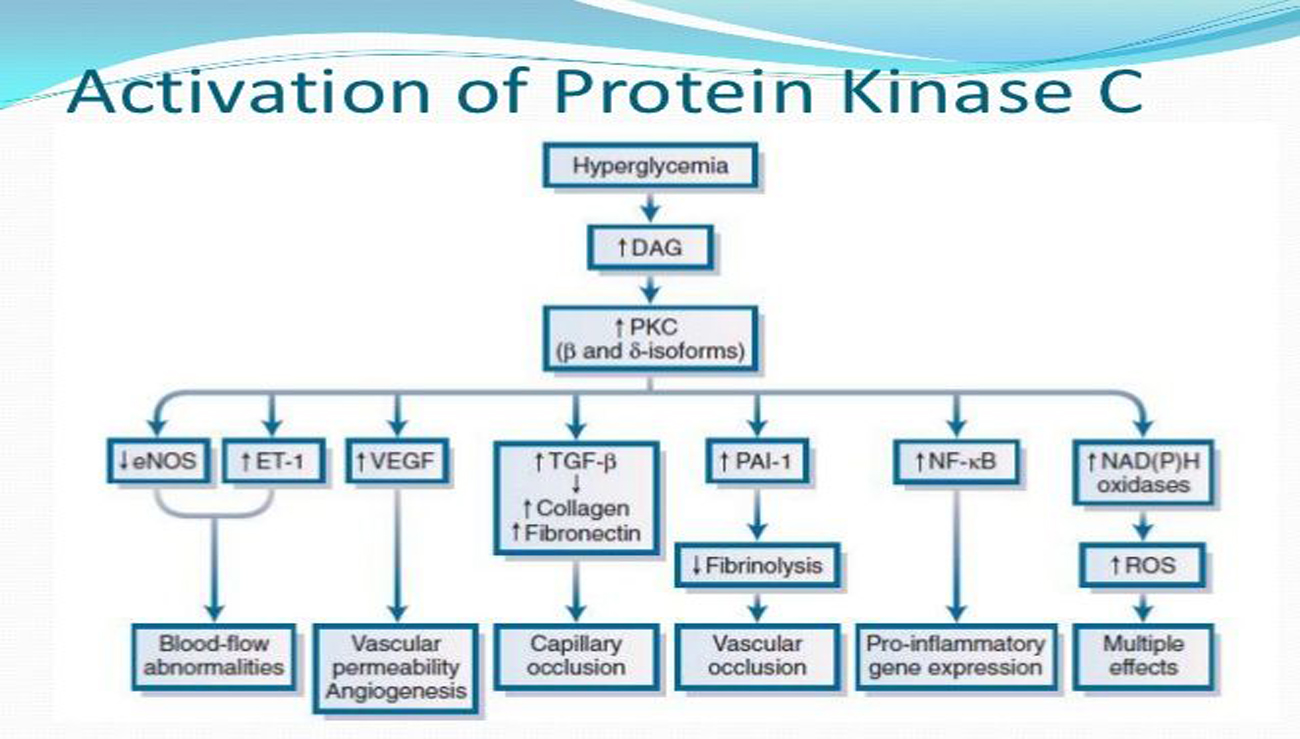
Diabetes is the most common and systemic disorder associated with hyperglycemia which is the significant factor in the development of micro- and macrovascular changes. Many mechanistic approaches i.e. activation of Protein kinase C, glycation end products production, hexosamine pathway and polyol pathway induce cellular damage and lead to the development of diabetic complications like nephropathy, neuropathy, retinopathy, and myopathy. One of the adverse effects of long-lasting hyperglycemia is activation of PKC (intracellular signaling enzyme) and has become a field of great research interest. Hence, in this review special emphasis is placed on microvascular complications which are due to activation of PKC. Clinical trials have also been conducted using selective PKC inhibitors and have shown positive results against hyperglycemia.
Author(s):
Simran, Amarjot Kaur Grewal, Sandeep Arora and Thakur Gurjeet Singh, Chitkara College of Pharmacy, Chitkara University, Punjab, India
Keywords:
Diabetes mellitus, Hyperglycemia, PKC, Microvascular complications, PKC inhibitors
URL:
https://jptrm.chitkara.edu.in/index.php/jptrm/article/view/127
References:
Adibhatla, R.M., Dempsey, R. and Hatcher, J.F. (2008). Integration of cytokine biology and lipid metabolism in stroke. Frontiers in bioscience: a journal and virtual library, 13, 1250. DOI: https://doi.org/10.2741/2759
Af Gennas, G.B., Talman, V., Aitio, O., Ekokoski, E., Finel, M., Tuominen, R.K. and Yli-Kauhaluoma, J. (2009). Design, synthesis, and biological activity of isophthalic acid derivatives targeted to the C1 domain of protein kinase C. Journal of medicinal chemistry, 52(13), 3969-3981. DOI: https://doi.org/10.1021/jm900229p
Aiello, L.P. (2002). The potential role of PKC β in diabetic retinopathy and macular edema. Survey of ophthalmology, 47, S263-S269. DOI: https://doi.org/10.1016/S0039-6257(02)00391-0
Aiello, L.P., Clermont, A., Arora, V., Davis, M.D., Sheetz, M.J., Bursell, S.E. (2006). Inhibition of PKC β by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Investigative ophthalmology & visual science. 2006 Jan 1, 47(1), 86-92. DOI: https://doi.org/10.1167/iovs.05-0757
American Diabetes Association (2005). The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: Initial results of the protein kinase C β inhibitor diabetic retinopathy study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005 Jul 1, 54(7), 2188-2197. DOI: https://doi.org/10.2337/diabetes.54.7.2188
Argoff, C.E., Cole, B.E., Fishbain, D.A. and Irving, G.A. (2006). Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. In Mayo Clinic Proceedings. 81(4), S3-S11. Elsevier. DOI: https://doi.org/10.1016/S0025-6196(11)61474-2
Arimura, T., Hayashi, T., Terada, H., Lee, S.Y., Zhou, Q., Takahashi, M., Ueda, K., Nouchi, T., Hohda, S., Shibutani, M. and Hirose, M. (2004). A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. Journal of Biological Chemistry, 279(8), 6746-6752. DOI: https://doi.org/10.1074/jbc.M311849200
Berger, R., Rotem-Yehudar, R., Slama, G., Landes, S., Kneller, A., Leiba, M., Koren-Michowitz, M., Shimoni, A. and Nagler, A. (2008). Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clinical Cancer Research, 14(10), 3044-3051. DOI: https://doi.org/10.1158/1078-0432.CCR-07-4079
Boone, T.K. (2013). liposomal formulation of rational combination of bioactive lipids for the treatment of leukemia (doctoral dissertation).
Brooks, B., Delaney-Robinson, C., Molyneaux, L. and Yue, D.K. (2008). Endothelial and neural regulation of skin microvascular blood flow in patients with diabetic peripheral neuropathy: effect of treatment with the isoform-specific protein kinase C β inhibitor, ruboxistaurin. Journal of diabetes and its complications, 22(2), 88-95. DOI: https://doi.org/10.1016/j.jdiacomp.2007.07.002
Caldwell, R.B., Bartoli, M., Behzadian, M.A., El‐Remessy, A.E., Al‐Shabrawey, M., Platt, D.H. and Caldwell, R.W. (2003). Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes/metabolism research and reviews, 19(6), 442-455. DOI: https://doi.org/10.1002/dmrr.415
Cameron, N.E., Gibson, T.M., Nangle, M.R. and Cotter, M.A. (2005). Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Annals of the New York Academy of Sciences, 1043(1), 784-792. DOI: https://doi.org/10.1196/annals.1333.091
Carrie, D. (2014). Transcriptional and Post-translational Regulation of Cytosolic Carbonic Anhydrase in Rainbow Trout (Oncorhynchus mykiss) and Zebrafish (Danio rerio) (Doctoral dissertation, Université d’Ottawa/University of Ottawa). https://doi.org/10.20381/ruor-3688
Chua, D.T., Sham, J.S. and Au, G.K. (2005). A phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral oncology, 41(6), 589-595. DOI: https://doi.org/10.1016/j.oraloncology.2005.01.008
Dirks, J., Remuzzi, G., Horton, S., Schieppati, A. and Rizvi, S.A.H. (2006). Diseases of the kidney and the urinary system. Disease control priorities in developing countries, 2, 695-706.
Evcimen, N.D. and King, G.L. (2007). The role of protein kinase C activation and the vascular complications of diabetes. Pharmacological research, 55(6), 498-510. DOI: https://doi.org/10.1002/9780470751503.ch23
Geraldes, P. and King, G.L. (2010). Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation research, 106(8), 1319-1331. DOI: https://doi.org/10.1161/CIRCRESAHA.110.217117
Gonçalves, C.M. (2013). Scavenger receptor cysteinerich proteins on the cross-roads between innate and adaptive immunity.
Joy, S.V., Scates, A.C., Bearelly, S., Dar, M., Taulien, C.A., Goebel, J.A., Cooney, M.J. (2005). Ruboxistaurin, a protein kinase C β inhibitor, as an emerging treatment for diabetes microvascular complications. Annals of Pharmacotherapy, 39(10), 1693-1699. DOI: https://doi.org/10.1345/aph.1E572
Karasu, Ç. (2010). Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. The open cardiovascular medicine journal, 4, 240. DOI: https://doi.org/10.2174/1874192401004010240
Kaschina, E. and Unger, T. (2003). Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood pressure, 12(2), 70-88. DOI: https://doi.org/10.1080/08037050310001057
Khera, T.K. (2006). Mesangial cell apoptosis and phagocytosis: the role of glucoseand transforming growth factor β-1. Cardiff University (United Kingdom).
Marsigliante, S., Muscella, A., Greco, S., Elia, M.G., Vilella, S. and Storelli, C. (2001). Na+/K+ ATPase activity inhibition and isoform-specific translocation of protein kinase C following angiotensin II administration in isolated eel enterocytes. Journal of endocrinology, 168(2), 339-346. DOI: https://doi.org/10.1677/joe.0.1680339
Martin, A., Komada, M.R. and Sane, D.C. (2003). Abnormal angiogenesis in diabetes mellitus. Medicinal research reviews, 23(2), 117-145. DOI: https://doi.org/10.1002/med.10024
Nagpal, M., Nagpal, K. and Nagpal, P.N. (2007). A comparative debate on the various anti-vascular endothelial growth factor drugs: pegaptanib sodium (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin). Indian journal of ophthalmology, 55(6), 437-439. DOI: https://doi.org/10.4103/0301-4738.36478
Naito, J., Koretsune, Y., Sakamoto, N., Shutta, R., Yoshida, J., Yasuoka, Y., Yoshida, S., Chin, W., Kusuoka, H. and Inoue, M. (2001). Transmural heterogeneity of myocardial integrated backscatter in diabetic patients without overt cardiac disease. Diabetes research and clinical practice, 52(1), 11-20. DOI: https://doi.org/10.1016/S0168-8227(00)00226-6
Nakamura, J., Kato, K., Hamada, Y., Nakayama, M., Chaya, S., Nakashima, E., Naruse, K., Kasuya, Y., Mizubayashi, R., Miwa, K. and Yasuda, Y. (1999). A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes, 48(10), 2090-2095. DOI: https://doi.org/10.2337/diabetes.48.10.2090
Nassar, H., Kantarci, A. and Van Dyke, T.E. (2007). Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontology, 2000, 43(1), 233-244. DOI: https://doi.org/10.1111/j.1600-0757.2006.00168.x
Nilsson, D. (2016). Forkhead Genes in Adipocytes and Podocytes. Department of Medical Biochemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy at University of Gothenburg.
Nishikawa, T., Edelstein, D., Brownlee, M. (2000). The missing link: a single unifying mechanism for diabetic complications. Kidney International, 58(S77), S26-S30. DOI: https://doi.org/10.1046/j.1523-1755.2000.07705.x
Noh, H. and King, G.L. (2007). The role of protein kinase C activation in diabetic nephropathy. Kidney International, 72(S106), S49-S53. DOI: https://doi.org/10.1038/sj.ki.5002386
Picchi, A., Capobianco, S., Qiu, T., Focardi, M., Zou, X., Cao, J.M. and Zhang, C. (2010). Coronary microvascular dysfunction in diabetes mellitus: a review. World journal of cardiology, 2(11), 377-390. DOI: https://doi.org/10.4330/wjc.v2.i11.377
Prabhakar, S.S. (2004). Role of nitric oxide in diabetic nephropathy. Seminars in nephrology, 24(4), 333-344. DOI: https://doi.org/10.1016/j.semnephrol.2004.04.005
Sakata, K., Kondo, T., Mizuno, N., Shoji, M., Yasui, H., Yamamori, T., Inanami, O., Yokoo, H., Yoshimura, N. and Hattori, Y. (2015). Roles of ROS and PKC-βII in ionizing radiation-induced eNOS activation in human vascular endothelial cells. Vascular pharmacology, 70, 55-65. DOI: https://doi.org/10.1016/j.vph.2015.03.016
Schramm, T.K., Gislason, G.H., Vaag, A., Rasmussen, J.N., Folke, F., Hansen, M.L., Fosbøl, E.L., Køber, L., Norgaard, M.L., Madsen, M. and Hansen, P.R. (2012). Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. European heart journal, 32(15), 1900-1908. DOI: https://doi.org/10.1093/eurheartj/ehr077
Sheetz, M.J. and King, G.L. (2002). Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. Jama, 288(20), 2579-2588. DOI: https://doi.org/10.1001/jama.288.20.2579
Shen, G.X., Way, K.J., Jacobs, J.R., King, G.L. (2003). Applications of inhibitors for protein kinase C and their isoforms. Protein Kinase C Protocols, 233, 397-422. DOI: https://doi.org/10.1385/1-59259-397-6:397
Spiegel, S., Foster, D. and Kolesnick, R. (1996). Signal transduction through lipid second messengers. Current opinion in cell biology, 8(2), 159-167. DOI: https://doi.org/10.1016/S0955-0674(96)80061-5
Tonneijck, L., Muskiet, M.H., Smits, M.M., Van Bommel, E.J., Heerspink, H.J., Van Raalte, D.H. and Joles, J.A. (2017). Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. Journal of the American Society of Nephrology, 28(4), 1023-1039. DOI: https://doi.org/10.1681/ASN.2016060666
Tripathi, B.K. and Srivastava, A.K. (2006). Diabetes mellitus: Complications and therapeutics. Medical Science Monitor, 12(7), RA130-147.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T., Mazur, M. and Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology, 39(1), 44-84. DOI: https://doi.org/10.1016/j.biocel.2006.07.001
Vinik, A. (2005). The protein kinase C-β inhibitor, ruboxistaurin, for the treatment of diabetic microvascular complications. Expert opinion on investigational drugs, 14(12), 1547-1559. DOI: https://doi.org/10.1517/13543784.14.12.1547
Wang, Y., Yin, O.Q., Graf, P., Kisicki, J.C., Schran, H. (2008). Dose and time dependent pharmacokinetics of midostaurin in patients with diabetes mellitus. The Journal of Clinical Pharmacology, 48(6), 763-75. DOI: https://doi.org/10.1177/0091270008318006
Wang, Y., Yang, H., Liu, H., Huang, J., Song, X. (2009). Effect of staurosporine on the mobility and invasiveness of lung adenocarcinoma A549 cells: an in vitro study. BMC cancer, 9(1), 174. DOI: https://doi.org/10.1186/1471-2407-9-174
Williamson, J.R., Chang, K., Frangos, M., Hasan, K.S., Ido, Y., Kawamura, T., Nyengaard, J.R., van Den Enden, M., Kilo, C. and Tilton, R.G. (1993). Hyperglycemic pseudohypoxia and diabetic complications. Diabetes, 42(6), 801-813. DOI: https://doi.org/10.2337/diab.42.6.801